科研绘图SCI画图作图学术杂志封面设计TOC示意图文章配图医学动画
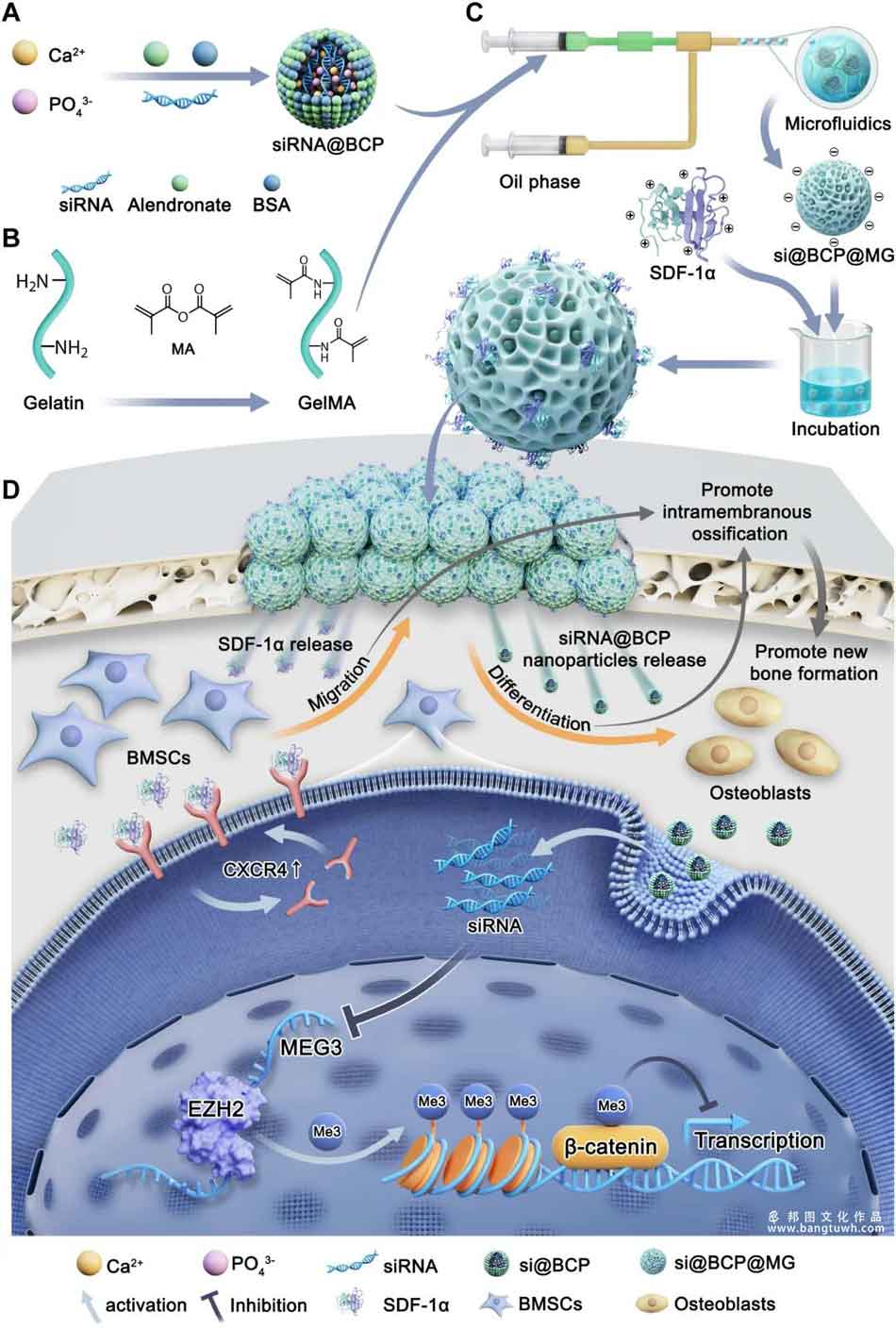




Critical-sized bone defects present significant clinical challenges due to insufficient stem cell recruitment, epigenetic suppression of osteogenesis, and inadequate mineralization. Among the epigenetic suppression mechanisms, upregulated MEG3 specifically recruits the epigenetic regulator EZH2 to block the transcription of β-catenin, a core gene for bone regeneration. To regulate MEG3 in vivo effectively, we used microfluidics to develop in situ continuous MEG3-silencing ossification micro-units (MSOMs) that integrate “material–gene–biofactor” tri-coupling into a unified biomaterial system. The MSOMs are nano-micro particles composed of amorphous calcium phosphate nanoparticles loaded with siRNA (si@BCP) in GelMA microgels loaded with stromal cell-derived factor-1α (SDF-1α).









