
科研绘图SCI画图作图学术杂志封面设计TOC示意图文章配图医学动画
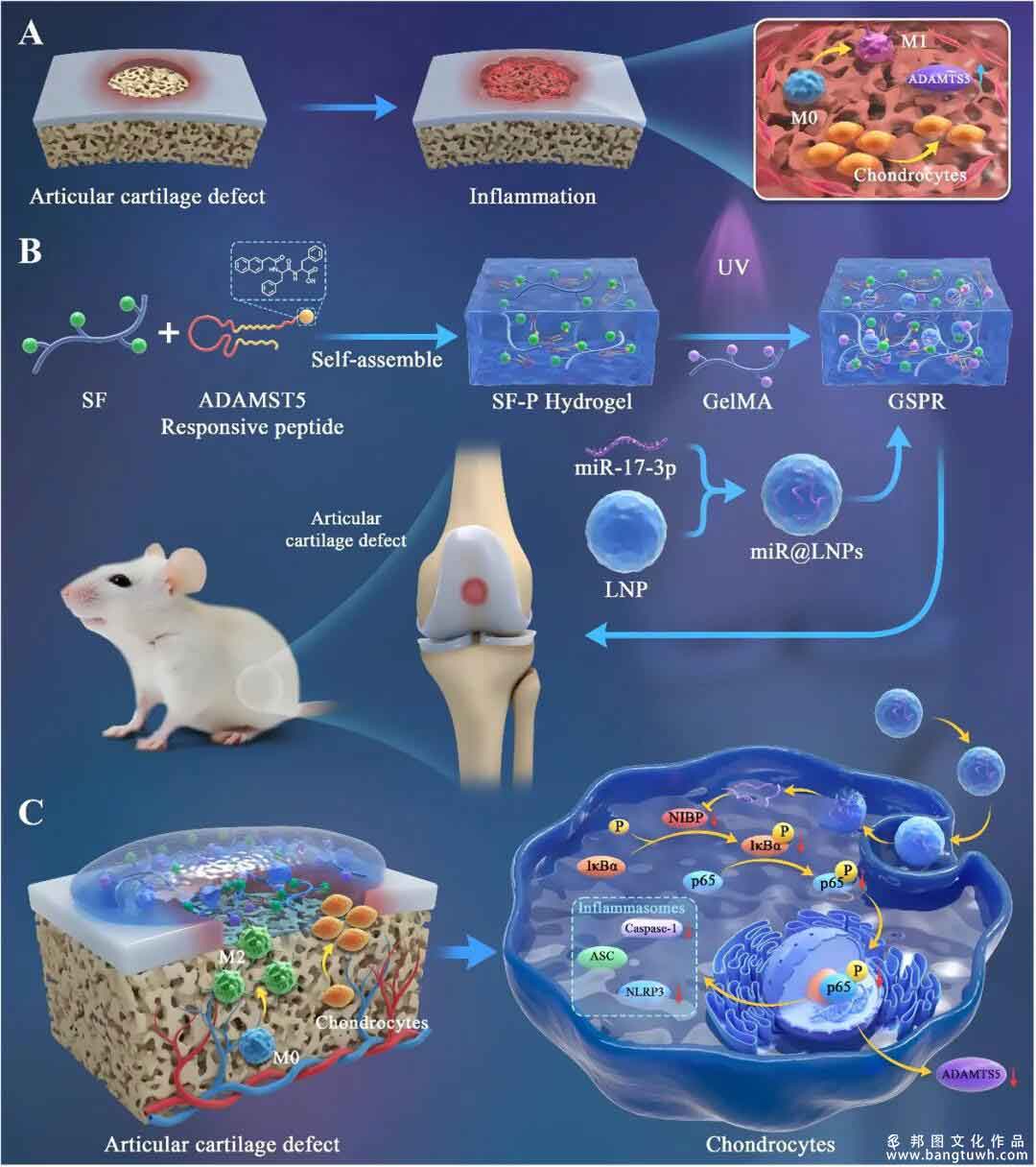




In situ articular cartilage (AC) regeneration is a meticulously coordinated process. Microfracture has been the most extensive clinical approach in AC repair, but it faces challenges such as matrix degradation, generation, and remodeling within a local inflammatory microenvironment. So far, it remains a challenge to establish a multistage regulatory framework for coordinating these cellular events, particularly the immune response and chondrocyte proliferation in microfracture-mediated AC repair microenvironments, which is crucial for promoting AC regeneration quality. At present, the excessive inflammatory response after microfracture can chronically activate the nuclear factor-κB (NF-κB) pathway, increasing production of matrix-degrading enzymes like matrix metalloproteinases (MMPs) and aggrecanases, which in turn accelerate cartilage matrix degradation and worsen the injury.








